Abstract
Introduction:
It is well known that haemostasis testing is influenced by many pre-analytical variables, such as storage time and temperature, that can affect the stability of coagulation factors and influence the results of coagulation assays. The stability of coagulation parameters may also depend on measurement principle and reagent used for analysis. The main aim of this study was to investigate the stability of haemostasis tests after storage of aliquoted plasma at room temperature (RT) for different time periods. Additionally, we investigated whether it is possible to accept a longer storage period at RT, compared to the current Clinical and Laboratory Standard Institute (CLSI) H21 A5 guidelines, without significant stability problems. Also, the influence of using different measurement principles and reagents on the stability of coagulation parameters was investigated by using two different fully automated haemostasis analysers with corresponding reagents.
Methods:
Blood samples from 20 healthy volunteers were obtained, immediately processed to platelet poor plasma (PPP) and aliquoted. Aliquots were stored at RT for 0h, 2h, 4h, 6h, 8h, 12h, 24h and 48h. Routine haemostasis parameters (PT, aPTT, fibrinogen and D-dimers) were performed on non-frozen plasma. Coagulation factors (FII, FV, FVII, FX, FVIII, FIX, FXI and FXII), von Willebrand factor antigen (vWF:Ag) and von Willebrand factor activity (vWF:Act) were performed batch-wise on frozen plasma aliquots.
All these parameters were determined by two analysers, STA-R Max® (Diagnostica Stago®, S.A.S., France) using a viscosity-based principle and ACL-TOP® 350CTS (Instrumentation Laboratory, Werfen, Bedford USA) using a turbidimetric measurement principle. Also analysis of PT and aPTT were performed by multiple reagents. VWF:Ag and vWF:Act were determined by the AcuStar® (Instrumentation Laboratory, Werfen, Bedford USA) using a chemiluminescence technique.
Statistically significant differences compared to the baseline results were defined by a Friedman repeated measures analysis of variance and a post hoc Wilcoxon signed rank test with bonferroni adjustment. A clinically relevant change in coagulation assay value compared to the initial measurement (analysis at 0h storage at RT) was denoted as a percentage change of > 10 % according to the 99% confidence interval (CI).
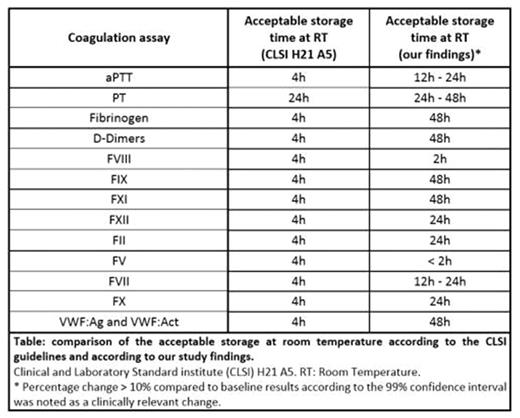
Results:
For both analysers, a decrease in factor activity of > 10% (according to the 99% CI) was observed for FV after 2h, FVIII after 4h and for FII and FX after 48h of storage at RT. A clinically significant decline of FVII activity, determined by ACL-TOP®, was observed at 24h storage at RT (99% CI: -13.3% - -0.7%). According to the 99% CI a clinically relevant decrease of FVII activity was seen after 48h of storage with mean percentage changes of 9.5% and 21.1% for STA-R Max® and ACL-TOP® respectively. A statistically significant and clinically relevant increase in FXII activity was seen after 48h storage of aliquoted plasma at RT.
For aPTT, a statistically significant difference was seen after 6h storage and mean percentage changes of > 10% were observed after 48h of storage at RT for all three aPTT reagents used. Statistically significant differences, but no clinically relevant changes were observed after a storage time of 48h for PT, fibrinogen and FIX. For D-Dimers, FXI, vWF:Ag and vWF:Act no statistically significant nor clinically relevant differences were observed after a storage period of 48h.
Conclusions:
Some differences between measurements by STA-R Max® and ACL-TOP® could be explained by the difference in reagent used for analysis. Overall, compared to the stated time limits given by the current CLSI guidelines, for most coagulation parameters investigated in this study a longer storage period could be accepted. Time intervals for FVIII and FV dosage were shorter than recommended by the CLSI guidelines. For PT determination our findings were consistent with the current CLSI guidelines.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal